Making the Case For ORP
What ORP is and when and how to use it instead of free chlorine
Author: Heather Rekalske
Though the measurement of free chlorine concentration is often indicated for the disinfection of water and disinfectant byproduct control, there is a better way. Because free chlorine works through oxidation, ORP instrumentation can be used to monitor and control its effectiveness. ORP measures the actual oxidation power of the solution, specifically the strength and number of oxidation and reduction reactions in solution, this yields a clear picture of the efficacy of the chlorine present, regardless of the concentration or ratio of chlorine species in solution.
Measuring ORP directly reflects the sanitizing power of free chlorine or any other oxidizing or reducing chemicals. The measurement of ORP is precise, empirical and requires no user interpretation, making it ideal for water quality and industrial process control.
What is ORP?
ORP is the acronym for Oxidation Reduction (REDOX) Potential. ORP is a differential measurement of the mV potentials built up when electrodes are exposed to solutions containing oxidants and reductants. ORP describes the net magnitude and direction of the flow of electrons between pairs of chemical species, called REDOX pairs. In a REDOX pair, one chemical loses electrons while the other chemical gains electrons. The chemical in the REDOX exchange that acquires electrons is called the oxidant (HOCL, OCl-, ClO2, bromine, hydrogen peroxide, etc.). The chemical in the REDOX exchange that gives up electrons is called the reductant + (Li, Mg2, Fe2, Cr2, etc.). Oxidants acquire electrons through the process of reduction, i.e., they are reduced. Reductants lose their electrons through the process of oxidation, i.e., they become oxidized.
How is ORP measured?
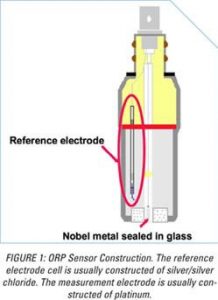 ORP sensors are two electrochemical half-cells: A measurement electrode in contact with the solution being measured and a reference electrode in contact with a reservoir of the highly concentrated salt solution.
ORP sensors are two electrochemical half-cells: A measurement electrode in contact with the solution being measured and a reference electrode in contact with a reservoir of the highly concentrated salt solution.
When the solution being measured has a high concentration of oxidizers, it will accept more electrons than it loses so that the measurement electrode develops a higher electrical potential than the reference electrode. A voltmeter placed in line with the two electrodes will display this difference in potential between the two electrodes. Once the entire system reaches equilibrium, the resulting net potential difference represents the ORP. A positive reading indicates an oxidizing solution, and a negative reading indicates a reducing solution. The more positive or negative the value, the more powerful the oxidants or reductants, the greater their concentrations or both.
What does ORP measure?
ORP can be used to determine the efficacy of chemical disinfectants that work via the oxidation or reduction of the structures of microbial contaminants. For example, chlorine, an oxidant, will strip electrons from the negatively charged cell walls of some bacteria. Because ORP measures the total chemical activity of a solution, ORP measures the total efficacy all oxidizing and reducing disinfectants in solution: Hypochlorous acid, monochloramine, dichloramine, hypobromous acid, sodium hypochlorite, UV, ozone, peracetic acid, bromochlorodimethylhydantoin, etc.
ORP indicates the effectiveness of only those disinfectants that work through oxidation and reduction. ORP cannot be used to detect the presence of any one particular chemical or chemical species. Nor can it alone be used to determine the concentration of a known species of chemical in solution, which means that although ORP is the best way to know whether or not your sanitizer is working, it can’t tell you how much or what kind of sanitizer is working.
What factors affect ORP measurement?
While the accuracy of ORP sensors is relatively stable, which is why they do not require calibration, there are factors that affect their response time. Changes in temperature can affect response times by altering the kinetic rates of the reactions being measured, for example. Low temperatures reduce the kinetic rates and lengthen sensor response times.
The condition of the electrode will also alter response times by changing the “exchange current density” (the number of electrons exchanged per unit area of exposed electrode). The lower the exchange current density, the more sluggish the sensor response. The typical measurement electrode is made from pure platinum (Pt) because it is a noble metal and, therefore, highly unreactive, i.e., the potential being measured is most likely due to the activity of the chemicals in the water and not reactions between the solution and the Pt itself. Even though Pt is a noble metal, it will form a thin oxide layer on the surface of the platinum when exposed to dissolved oxygen. This oxide layer facilitates the ORP measurement when it is very thin, one molecule thick, by attracting, or “absorbing,” hydrolyzed oxidant or reductant molecules to the surface of the electrode.
Unfortunately, when the oxide layer becomes more than one molecule thick, the resulting lowered exchange current density offsets this benefit. Also, the adsorbed molecules cause a “memory effect.” If a sensor is placed in a less oxidizing solution after measuring a more oxidizing solution, it can take a very long time for the sensor to equilibrate to the new sample. Though the sensor response time is much slower, the final ORP reading will be the same.
ORP electrodes never require recalibration because there is no drift in zero point (as is the case with pH sensors). Any deviation from expected readings is most likely due to surface contamination of the electrodes or buildup of the oxide layer, both of which can easily be remedied by cleaning with a light abrasive, such as Softscrub®. Exposing the sensor to an “ORP conditioning solution” will help reduce the memory effect due to adsorption.
Can ORP be used as a surrogate parameter for free chlorine?
Yes. ORP measures the oxidizing power and, therefore, the actual residual sanitizing strength of the solution being tested. Simply counting how much chlorine is present is misleading because certain changes in water chemistry, such as pH or the addition of cyanuric acid, dramatically alter the oxidizing power of chlorine and, therefore, its efficacy, without changing how much chlorine is present.

When correlated with established disinfection control parameters, measurements and bacterial plate counts, this type of measurement gives a very accurate picture of the sanitizing activity. For this correlation to be valid, the water undergoing treatment must be characterized so that all chemical constituents are known. The pH and temperature values should be reported and held constant. ORP will report an empirical value or a hard number that indicates how active the sanitizer is. However, you have to make certain that microbial contamination is responding to the treatment. Once a correlation is established in a stable system, ORP is a very efficient and effective way to monitor microbial control.
ORP has long been used in bathing waters as the only means for automatic chemical dosing. In fact, the World Health Organization (WHO) suggests an ORP value of between 680-720 mV, depending on the sensor and the particular context, for safe bathing water. In the disinfection of drinking water, an ORP value of ~800 mV is required for oocyst inactivation.
For the purpose of pretreatment screening to detect chlorine levels prior to contact with chlorine-sensitive RO membranes, influent must first be screened to determine which chemicals besides chlorine are present that contribute to the ORP value. With these interferants characterized and pH and temperature held constant, ORP can be correlated to specific sanitizer concentrations, such as chlorine, in their known forms. Some manufacturers of RO membranes and other water quality treatment equipment will also specify an ORP tolerance value for prescreening and influent control. The same holds true of effluent screening.
Why ORP?
ORP is a faster, simpler empirical measurement than titration with DPD or other methods, and in many cases, it gives the most accurate picture of the effect of all oxidizing and reducing chemicals in solution. No in-depth knowledge or training is required to obtain accurate, repeatable results. User error is virtually eliminated because ORP readings require no subjective, visual interpretation, nor do they require calibration.
Using ORP disinfectant control can be automated because the measurement produces an electrical signal that can trigger switches when outside established control parameters. And ORP sensors are relatively low-maintenance. If you’re not using ORP to monitor and control chemical additions that work through REDOX, you should. You’ll save yourself time, hassle and money.
Heather Rekalske is a technical writer for the Myron L Company, a privately held company whose core is researching and developing effective and innovative solutions for the water quality industry. Myron L manufactures a broad spectrum of water quality instrumentation, including reliable, cost-effective monitor/controllers as well as precision digital and analog handheld meters. The most exciting new development in the handheld line is the FCE function, which accurately measures free chlorine using accelerated ORP readings.

